Contact Us
Have a question? If you can’t find what you’re looking for, contact our customer service team via contact us page, or call (02) 8062 9800.
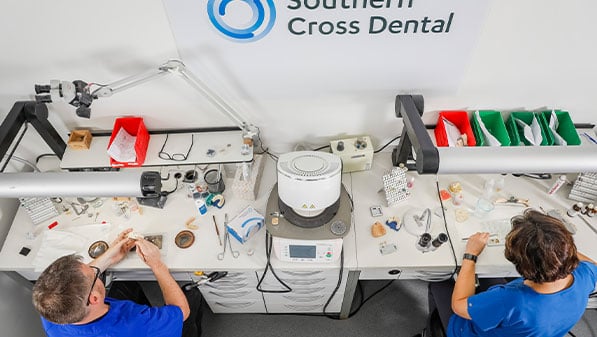
In Australia, most dental laboratory products are categorised as a ‘medical device’ for regulatory purposes. This means that the manufacture, export and supply is regulated by the Therapeutic Goods Administration (TGA).
We adhere to the highest levels of quality management to ensure that our products are made in compliance with all government regulations. The safety and reliability of our products sets the standard for high-quality dental products.
Contact Us
At Southern Cross Dental, we source all our raw materials and components from internally evaluated and approved internationally reputable suppliers: Ivoclar Vivadent, Dental Direkt, Argen and Asiga, to name a few.
All product critical materials are listed on the Australian Register of Therapeutic Goods, managed by ourselves or the Australian sponsor. This standard is maintained by our quality processes.
If you would like to see data sheets or more information for any of our materials, please get in touch. Our systems ensure identification and traceability from purchase, through manufacture, all the way to sale.
Contact Us
Here at Southern Cross Dental, we meet the regulatory obligations set by the Therapeutic Goods Administration (TGA). The TGA was established to ‘safeguard and enhance the health of the Australian community through effective and timely regulation of therapeutic goods’. The product portfolio contains classified Patient-Matched Devices. This means:
The TGA has been notified of all patient matched medical devices that we produce, as part of the transition phase.
All cases are manufactured on instruction from an authorised health professional, using materials that are listed on the ARTG.
All the products of Southern Cross Dental are manufactured under a Quality Management System, certified under ISO1385 Medical Devices by a third party notified body.
Prior to the transition phase ending in 2029, we will list certain patient matched medical devices directly on to the ARTG.
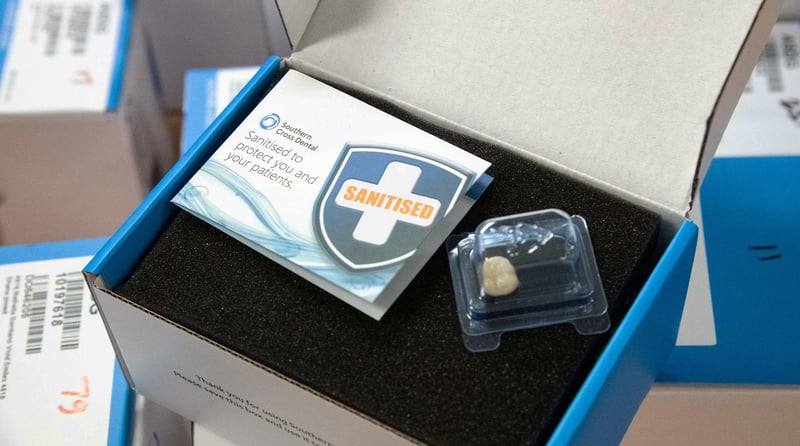
More than ever, it is so important to provide clean and safe practices, and we operate stringent decontamination procedures at Southern Cross Dental. This means that all cases are processed on arrival at our facilities, and returned to you with confidence. Our Standard Range production facilities have received ISO 13485:2016 for medical device manufacturing. All products are sanitized with FDA-approved disinfectants.
Our Australian sites operate under an Infection Control Management Plan that is compliant with Dental Board of Australia, Australian Dental Association Guidelines and Australian/New Zealand Standards.
In our Standard Range, product manufacture is also governed by protocols aligned with The Guideline for Disinfection and Sterilization in Healthcare Facilities (Centre for Disease Control, 2008).
Interested to see our infection control practices? Watch here.
Contact us for more information about our best industry practice for safety and compliance.

You can rest assured that all products supplied by Southern Cross Dental are manufactured under a Quality Management System (QMS) which is certified under ISO13485 Medical Devices. This is the gold standard for the manufacture of Medical Devices.
Our QMS raises the standard of how we manufacture dental medical devices for you, the dentist. It ensures that we have clear and robust processes for material selection, equipment maintenance, staff training, and rigorous quality control.
Importantly, is means that for every medical device that we make for you, we can trace the materials and components used, and be confident in providing you with a high quality restoration. Our QMS also includes customer feedback loops, so you can be confident that when you provide us with comments, we learn from that and feed that back to improve future production.
The QMS is audited by a third party notified body on an annual basis, and you can view are current audit certificate here.